Coordinators:
Alex Abela
John Cant
Mars Muusse
Index ORG
ring projects
PDF's
1cy June
1cy July
1cy Aug
1cy Sept
1cy Oct
1cy Nov
1cy Dec
2cy Jan
2cy Feb
2cy March
2cy April
2cy May
2cy June
2cy July
2cy Aug
2cy Sept
2cy Oct
2cy Nov
2cy Dec
3cy Jan
3cy Feb
3cy March
3cy April
3cy May
3cy June
3cy July
3cy Aug
3cy Sept
3cy Oct
3cy Nov
3cy Dec
4cy / sub-ad Jan
4cy / sub-ad Feb
4cy / sub-ad March
4cy / sub-ad April
4cy / sub-ad May
4cy / sub-ad June
4cy / sub-ad July
4cy / sub-ad Aug
4cy / sub-ad Sept
4cy / sub-ad Oct
4cy / sub-ad Nov
4cy / sub-ad Dec
adult Jan
adult Feb
adult March
adult April
adult May
adult June
adult July
adult Aug
adult Sept
adult Oct
adult Nov
adult Dec |
1st cycle (juvenile): June
GEOGRAPHIC VARIATION AND EVOLUTION IN THE CALIFORNIA GULL (LARUS CALIFORNICUS)
JOSEPH R. JEHL, JR.
- The Auk 104: 421-428. July 1987 -
Sea World Research Institute / Hubbs Marine Research Center, 1700 South Shores Road, San Diego, California 92109 USA
ABSTRACT
The California Gull (Larus californicus), currently regarded as a monotypic species, is separable into a small, dark-mantled race (L. c. californicus) that breeds primarily in the Great Basin of the United States, and a larger, paler race (L. c. albertaensis) from the Great Plains of the north-central U.S. and south-central Canada. The breeding ranges of these two races, previously disjunct, have expanded recently, and a zone of secondary contact seems to be forming east of the Rocky Mountains in the northern United States.
The California Gull (Larus californicus) has a discontinuous breeding range in the western United States and Canada, extending from Great Slave Lake, N.W.T. (62øN) south to San Francisco Bay and Mono Lake, California (38øN) and east to the Dakotas (A.O.U. 1983, pers. obs.). Except for a brief study by Zink and Winkler (1983), who found only minor size and genetic differences between gulls from Mono Lake and Great Salt Lake, Utah, there has been no effort to investigate geographic variation in this species, which is pronounced.
METHODS
Museum specimens used in this analysis were taken in the breeding range between 1 April and 15 August. Because some gulls are still migrating to inland breeding stations in April, and because adults begin to move coastward by the last days of July, samples from western parts of the range may have included migrants. I took standard measurements on length of the exposed culmen, flattened wing and tarsus, depth of the bill at the gonys, and body mass. The large sample from Mono Lake is based mainly on birds found freshly dead on nesting islets; a representative series was preserved as museum specimens. Some California Gulls begin to show extensive wear of the primaries by March. Specimens whose wing length could not be determined accurately were excluded from the analysis. Additional size and mass data were extracted from the literature. I also noted the patterning of the primaries and the color of the mantle. Although variation in the latter was evident, I could not measure it consistently because of the generally soiled condition of most specimens.
For analysis of size variation I separated mensural data by sex and used principal components analysis (BMDP4M; Dixon 1983) to test for patterns of variation. The analysis showed that the gulls fell into two distinct assemblages. I then used stepwise discriminant function analysis (BMDP7M; Dixon 1983) to determine which variables contributed to the separation of groups, using locality as the grouping variable.
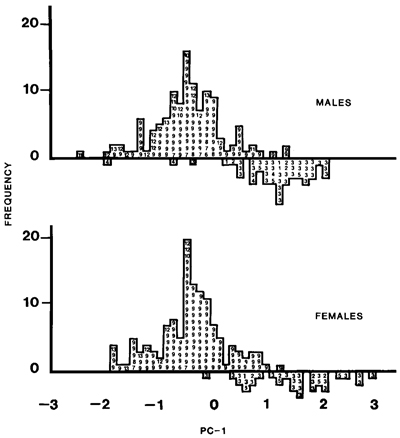
Fig. 2. Plot of first principal component of male and female California Gulls. Numbers refer to localities: ! = Northwest Territories, 2 = Saskatchewan, 3 = Alberta, 4 = Montana, 5 = North Dakota, 6 = Washington, 7 = Oregon, 8 = Idaho, 9 = Mono Lake, California, 10 = other California, 11 = Nevada, 12 = Utah, and 13 = Colorado.
Click image for larger size.
|
RESULTS
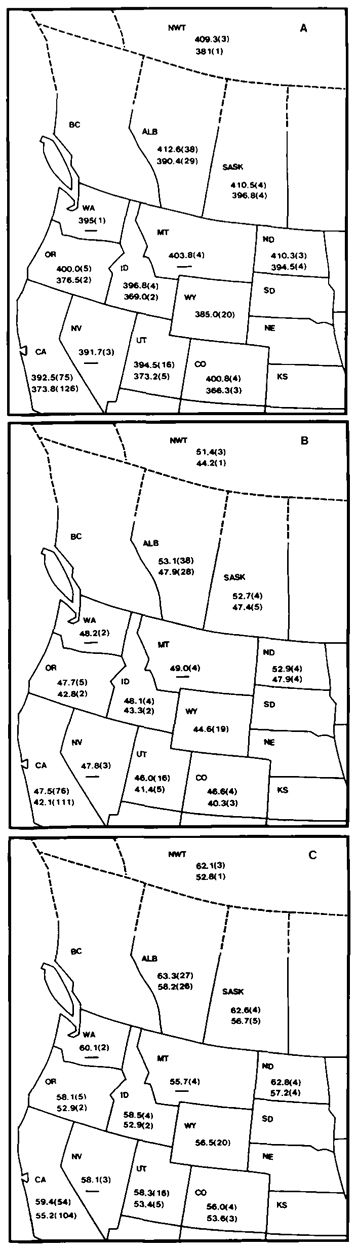
Although California Gulls are well represented in museum collections, material from the breeding grounds is scanty. Even so, considerable geographic variation in size is obvious (Appendix). A preliminary comparison of the only two large samples, from Alberta and Mono Lake, showed that Alberta birds were significantly larger in culmen, wing, tarsus, and mass (t-test, P < 0.05); they also had a paler mantle. Simple inspection of the data showed that the size differences did not conform to a simple latitudinal gradient, as might be expected from Bergmann's rule; some of the largest birds were from North Dakota (48øN) and some of the smallest from Utah (41øN) (Fig. 1). Principal components analysis showed that 58% of the variation in females and 61% in males was explained by a single factor and that within each sex two assemblages could be recognized (Fig. 2). These assemblages corresponded broadly to physiographic provinces in western North America. Gulls from the northern Great Plains of the north-central United States, the prairie provinces of Canada, and the Northwest Territories were large and clustered with the Alberta sample. Those from the Great Basin of the western United States (Washington, Oregon, Nevada, Utah, and California), as well as those from nearby regions in Colorado, Wyoming, and Idaho, were small like those from Mono Lake. Although samples from many regions were very small, the available data did not reveal any indication of clinal variation.
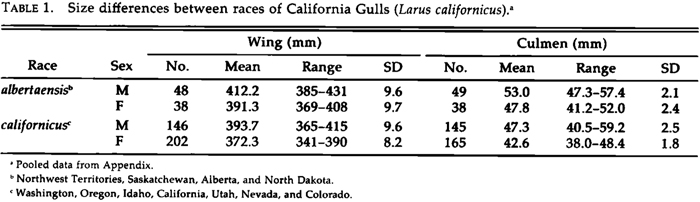
External differences between the Great Basin and Great Plains forms are greater than those characterizing most currently recognized races of North American birds. I describe them as subspecies because morphological and historical (see below) data indicate they have had separate evolutionary histories. The type specimen of the species (Amer. Mus. Nat. Hist. No. 46070) was taken near Stockton, California, and is an adult (cf. Greenway 1978) female of the Great Basin population, which becomes L. c. californicus. Its measurements are: exposed culmen, 43.7 mm; depth of bill at gonys, 15.8 mm; tarsus, 55.4 mm; wing, 380 mm. For the larger form I propose the name:
Larus californicus albertaensis ssp. nov.
Holotype. University of Michigan Museum of Zoology No. 224,653, adult male, from Frog Lake, Alberta, 53ø55'N, 110ø15'W; collected by Philip H. R. Stephey, 9 July 1985. Diagnosis. Distinguished from L. c. californicus by greater size, particularly in bill dimensions and body mass (Table 1, Fig. 3). Mantle paler gray, less bluish than in L. c. californicus, approaching or matching the paleness of L. argentatus (Fig. 4). Measurements of holotype: exposed culmen, 52.5 mm; depth of bill at gonys, 18.7 ram; tarsus, 62.6 ram; wing, 424 ram; mass, 809 g. Using stepwise discriminant function analysis for the entire sample, I could determine the racial identification of a bird of known sex for 87.9% of the females of L. c. albertaensis and 95.3% of the females of L. c. californicus, and for 87.0% of the males of L. c. albertaensis and 92;4% of the males of L. c. californicus (Fig. 5) by the following formulas, where a score >0 is albertaensis and a score <0 is californicus: for females, 0.36 culmen (mm) + 0.31 bill depth (mm) + 0.06 wing (mm) - 45.60; for males, 0.25 culmen (mm) + 0.15 tarsus (mm) + 0.05 wing (mm) -/- 40.21.
Range. Nests on lakes, often of slight to moderate alkalinity or salinity, in North (and South?) Dakota, Manitoba, Saskatchewan, Alberta, and the Northwest Territories (Fig. 6). Southern and western limits unknown, as the range of the species is changing rapidly. Presumably winters through the range of the species, mainly along the Pacific coast from British Columbia to Baja California.
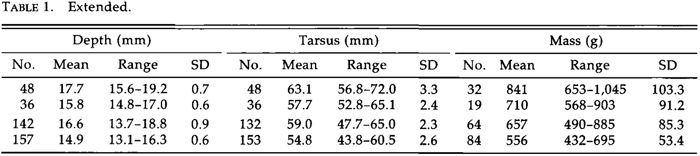
Variation. Larus c. albertaensis averages 5-12% larger than L. c. californicus in linear dimensions and 27% greater in body mass. All of these differences are highly significant (t-test, P < 0.0001). Indeed, females of albertaensis exceed male californicus in mean culmen length and mass. In addition, albertaensis has a paler mantle, and the gray areas on the inner vane of the primaries average larger and paler, although the latter differences are minor. The pattern of white markings ("mirrors") on the primaries of adults is variable (see Dwight 1925: figs. 115, 117, 119, 120), though not geographically. I found no differences in color or pattern in the downy and juvenile plumages of 10 gulls from Mono Lake and 10 from Beaverhill Lake that were hatched and reared in captivity. Among wild-taken birds, juvenile and subadult plumages were too variable for analysis. Juveniles from Mono Lake, for example, range from pale tan to dark brown in their general coloration, which may be modified dramatically by exposure to alkaline water and intense sunlight. Studies of allozyme variability revealed no diagnostic genetic differences between the two populations (Karl et al. 1987).
Specimens examined. The type series of 9 males and 3 females from Frog Lake and Beaverhill Lake, Alberta, is housed in the University of Michigan Museum of Zoology (2 skins), the Provincial Museum of Alberta (2 skins), and the San Diego Museum of Natural History (4 skins, 4 skeletons). Other specimens examined are in the museums acknowledged below. Tissue samples of both forms, including the type of L. c. albertaensis, have been deposited in the Museum of Zoology, Louisiana State University.
Etymology. Named for Alberta, Canada, province of the type locality.
 |
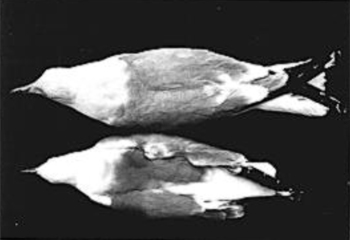 |
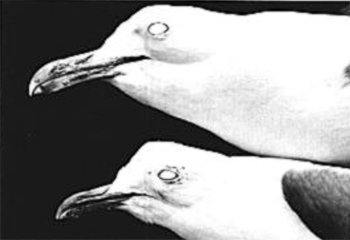
| Fig. 3. Extremes of geographic and sexual variation in bill dimensions in California Gulls. Top: L. c. albertaensis, male, Frog Lake, Alberta (UMMZ No. 226,654). Bottom: L. c. californicus, female, Mono Lake, California (SDNHM No. 44123). |
Fig. 4. Variation in body size and mantle color in California Gulls. Top: L. c. albertaensis. Bottom: L. c. californicus. Specimens as in Fig. 3. |
DISCUSSION
Evolution and subspeciation in the California Gull: a hypothesis. Rapid speciation among large gulls in the Northern Hemisphere is presumed to have resulted from the splitting of ancestral populations by events in the Pleistocene (Mayr 1963, Smith 1964). I postulate that when the California Gull stock was divided, one population (albertaensis) became isolated in the northern lakes on outwash plains near the margins of the glaciers. Until recently albertaensis bred mainly at lakes near the edge of the boreal forest (see Fig. 6). The other population (californicus) became isolated around lakes in the steppe-desert of the western United States, where the species is currently most abundant (Conover 1983).
In the pleniglacial period (ca. 12,500-15,000 yr B.P.) the refugium for the cold-desert flora characteristic of the modern Great Basin, and presumably for L. c. californicus, was in a small area of the present Mohave Desert (Wells 1983). As the climate ameliorated in the Holocene, steppe-desert conditions shifted north into the Great Basin, where extensive lakes of varied hydrology provided breeding habitat for gulls (Hubbs and Miller 1948, Hubbs et al. 1974). I infer that the absence of suitable nesting habitat in the High Plains helped isolate the Great Plains and Great Basin gulls, which as recently as 1915 were 700 km apart (Cooke 1915; Fig. 6). Shortly thereafter a major range expansion began, abetted by the creation of nesting habitat. By 1932 the Great Basin birds had bred in Washington (Decker and Bowles 1932), and some had spread across the Rockies, forming colonies in Wyoming and Colorado (Bailey and Niedrach 1965), a process that continues today (Findholt 1986, C. Chase pets. comm.). The regularity of nonbreeders summering in New Mexico suggests that colonization may be imminent (J.P. Hubbard pets. comm.); at least 5 of 6 specimens taken there in the summer of 1981 represent L. c. californicus. Data for the Great Plains birds are scanty, but the recent establishment of a colony in South Dakota (Harris 1982) suggests that their range, too, is expanding.
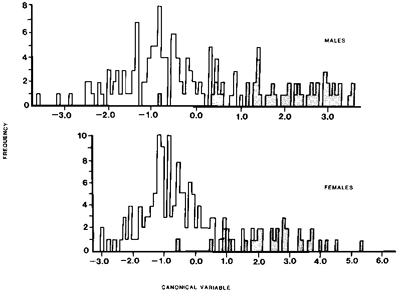
Fig. 5. Results of discriminant function analysis. Stippled areas refer to samples from the range ascribed to L. c. albertaensis, open areas to range of L. c. californicus.
|
Between 1930 and 1980 the U.S. population increased from 101,000 gulls in 15 colonies to 276,000 in 80 colonies, and the hiatus that existed in the High Plains began to fill (Conover 1983). The increased abundance and range expansion apparently resulted in a zone of secondary contact east of the Continental Divide, with birds in that region being derived from breeding areas to the northeast and from the southwest. The small sample from Montana (4 males only), for example, includes two birds that clump with the Great Basin birds, one with the Great Plains birds, and one that is intermediate (Fig. 2).
The ancestry of the California Gull is uncertain. Most authors have considered it a member of the Herring Gull complex (Stegmann 1934, Fisher and Lockley 1954, A.O.U. 1983), and among extant forms it is reminiscent of Thayer's Gull (L. thayeri) in its small size, dark iris, relatively dark mantle, juvenile and subadult plumages, east-west migration route, and west coast wintering range. Schnell (1970, pers. comm.), on the other hand, using only specimens that I would classify as L. c. californicus, found that the species clustered more closely with the smaller Ring-billed (L. delawarensis) and Mew (L. canus) gulls than with the Herring Gull. While I suspect a reanalysis that included large specimens from the Great Plains might lead to a different result, I doubt any conclusions will be fully satisfactory until the possible relationship of L. californicus to two small Pleistocene species, L. oregonus and L. robustus, is considered. Those species are known only from inland localities in the Great Basin (Howard 1946, Jefferson 1985), the area now inhabited by L. c. californicus.
Sampling procedures. The paucity of preserved material from many parts of the gulls' range is deplorable but easily remedied. Collecting east of the Continental Divide, especially, is needed to document current morphological variation more precisely and to determine whether the differences described above will be swamped or reinforced as the species continues its increase and range expansion.
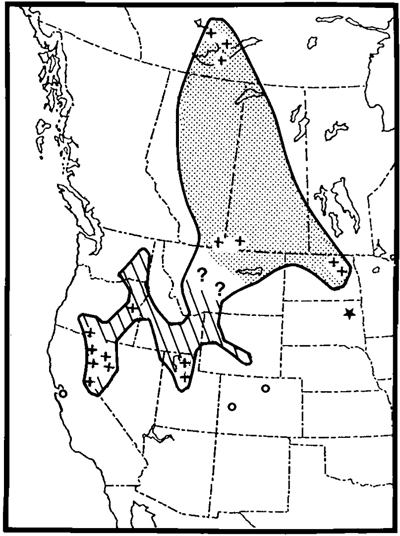
Fig. 6. Breeding range of California Gull, based on Conover (1983), Findholt (1986), Godfrey (1966), Harris (1982), C. Chase, III (pers. comm.), and original observations. The main range of L. c. californicus is hatched; outlying colonies are indicated by open circles. The range of L. c. albertaensis is stippled; a colony in South Dakota ★) is presumed of be of this race. For precise location of U.S. colonies see Conover (1983). Colonies known to Cooke (1915) are indicated by crosses.
|
Considerable sampling bias in currently available material is manifested by the sex ratios of specimens used in this study, which vary among localities (see Appendix); indeed, females were unrepresented in some samples. The high incidence of males (109 males vs. 69 females) from localities other than Mono Lake probably stems from their greater aggressiveness and likelihood to approach within shotgun range (Butler and Janes-Butler 1982, Burger 1983). By contrast, the high incidence of females in the Mono Lake (72 males vs. 124 females) sample, derived largely from birds dying of trauma or predation in the colony, reflects the females' greater risk of mortality early in the nesting season (Jehl and Chase 1987, Jehl MS). These contradictory ratios illustrate further the inherent risks in drawing inferences about social structure from the sex ratios of preserved specimens, when bias in collection or preservation is not, or cannot be, known (Burger 1983; cf. Conover and Hunt 1984).
I examined collections in the Museum of Vertebrate Zoology, University of California, Berkeley; California Academy of Sciences; University of California, Los Angeles; Los Angeles County Museum of Natural History; U.S. National Museum of Natural History, Field Museum of Natural History; and San Diego Museum of Natural History. Information on other collections was provided by C. M. White (Brigham Young University, University of Utah), R. M. Mengel (University of Kansas Museum of Zoology), K. C. Parkes (Carnegie Museum of Natural History), R. W. Starer (The University of Michigan Museum of Zoology), L. Cancade and P. Stepney (Provincial Museum of Alberta), M. Gillman (California Academy of Sciences), G. Schnell (University of Oklahoma), M. R. Browning (U.S. National Museum of Natural History), C. Chase, III (Denver Museum of Natural History), T. Miller (British Columbia Provincial Museum), and L. Oring (University of North Dakota). G. McCaskie, P. Stepney, S. Findholt, and C. Chase helped in many ways. Special thanks are due S. I. Bond for continual assistance in all phases of this and complementary research. Computer programs were run at the San Diego State University Computer Center. The manuscript was improved by the comments of T. R. Howell, S. A. Karl, R. W. Starer, R. W. Zink, G. D. Schnell, A. H. Brush, D. M. Power, M. Conover, and B. L. Monroe, Jr. This research was supported by grants from the Los Angeles Department of Water and Power.
LITERATURE CITED
AMERICAN ORNITHOLOGISTS' UNION. 1983. Check-list of North American birds, 6th ed. Washington, D.C., Amer. Ornithol. Union.
BAILEY, g. M., & R. J. NIEDRACH. 1965. Birds of Colorado. Denver, Colorado, Denver Museum of Natural History.
BEHLE, W. H. 1958. The bird life of Great Salt Lake. Salt Lake City, Univ. Utah Press.
BURGER, J.1983. Determining sex ratios from collected specimens. Condor 85: 503.
BUTLER, g. G., & S. JANES-BUTLER. 1982. Territoriality and behavioral correlates of reproductive success of the Great Black-backed Gulls. Auk 99: 59-66.
CONOVER, M.R. 1983. Recent changes in Ring-billed and California gull populations in the western United States. Wilson Bull. 95: 362-383.
--, & G. L. HUNT, JR. 1984. Female-female pairing and sex ratios in gulls: an historical perspective. Wilson Bull. 96: 619-625.
COOKE, W. W. 1915. Distribution and migration of North American gulls and their allies. U.S. Dept. Agr. Bull. 292.
DECKER, F. R., & J. H. BOWLES. 1932. Two new breeding records for the state of Washington. Murrelet 13: 53.
DIXON, W. J. (Ed.). 1983. BMDP statistical software. Los Angeles, Univ. California Press.
DWIGHT, J. 1925. The gulls (Laridae) of the world: their plumage, moults, variations, relationships and distribution. Bull. Amer. Mus. Nat. Hist. 52: 63-408.
FINDHOLT, S. 1986. Status and distribution of California Gull nesting colonies in Wyoming. Great Basin Natur. 46: 128-133.
FISHER, J., & R. M. LOCKLEY. 1954. Sea-birds. An introduction to the natural history of the sea-birds of the North Atlantic. Boston, Houghton Mifflin Co.
GODFREY, W. E. 1966. The birds of Canada. Ottawa, Queen's Printer.
GREENWAY, J. 1978. Type specimens of birds in the American Museum of Natural History. Bull. Amer. Mus. Nat. Hist. 161: 1-305.
HARRIS, B. 1982. First nest record for California Gulls in South Dakota. South Dakota Bird Notes 34: 42. HOWARD, H. 1946. A review of the Pleistocene birds from Fossil Lake, Oregon. Carnegie Inst. Washington Publ. 551: 141-195.
HUBBS, C. L., & R. R. MILLER. 1948. The zoological evidence: correlation between fish distribution and hydrographic history in the desert basins of western United States. Pp. 17-166 in The Great Basin, with emphasis on Glacial and Postglacial times. Bull. Univ. Utah 38 (Biol. Ser. 10). ,
--, & L. C. HvB•s. 1974. Hydrographic history and relict fishes of the north-central Great Basin. Mem. California Acad. Sci. 7: 1-259.
JEFFERSON, G. T. 1985. Review of the Late Pleistocene avifauna from Lake Manix, central Mojave Desert, California. Contrib. Sci., Los Angeles County Mus. No. 362.
JEHL, J. R., JR., & C. CHASE, III. 1987. Foraging patterns and prey selection by avian predators: a comparative study in two colonies of California Gulls. Stud. Avian Biol. 10. KARL, S. A., R. M. ZINK, & J. R. JEHL, JR. 1987. A1- lozyme analysis of the California Gull (Larus californicus). Auk 104: in press.
MAYR, E. 1963. Animal species and evolution. Cambridge, Massachusetts, Belknap Press, Harvard Univ.
SCHNELL, G. D. 1970. A phenetic study of the suborder Lari (Aves) II. Phenograms, discussion and conclusions. Syst. Zool. 19: 264-302.
SMITH, J. E., & K. L. DIEM. 1972. Growth and development of young California Gulls (Larus californicus). Condor 74: 462-470.
SMITH, N. G. 1964. Evolution of some arctic gulls (Larus): an experimental study of isolating mechanisms. Ornithol. Monogr. No. 4.
STEGMANN, g. 1934. Ueber die Formen der grossen Mowen ("subgenus Larus") und ihre gegenseitigen Beziehungen. J. Ornithol. 82: 340-380.
VERMEER, K. 1970. Breeding biology of California and Ring-billed gulls: a study of ecological adaptation to the inland habitat. Can. Wildl. Serv. Rept. Ser. No. 12.
WELLS, P.V. 1983. Paleobiogeography of montane islands in the Great Basin since the last glaciopluvial. Ecol. Monogr. 53: 341-382.
ZINK, R. g., & D. W. WINKLER. 1983. Genetic and morphological similarity of two California Gull populations with different life history traits. Blochem. Syst. Ecol. 11: 397-403. |
Fig. 1 below. Mean dimensions of (A) wing length, (B) culmen length, and (C) tarsus length of California Gulls. For each state or province, data for males are listed above those for females. The sample for Wyoming was based on unsexed individuals. Sample sizes are in parentheses. |
 California Gull californicus
California Gull californicus





